Life Sciences

No other Industry has more stringent demands than the Life Sciences Industry. IES Lean Systems Ltd. has worked with many of the major companies in Bulk and Dispensary applications. We have experience in:
21CFR11 compliance
EBR (Electronic Batch Records) Electronic Signatures RFID (Radio Frequency Identification)
in Bulk Production
IES Lean Systems Ltd. have supplied:
DCS systems with Recipe Management
Batch Tracking and Reporting
Instrumentation and Inerting Systems
Life Science Examples:
21CFR11
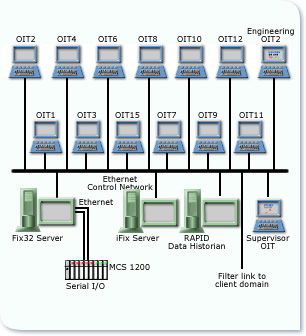
The existing Distributed Control System was revamped to provide an improved Operator Interface, enhanced Recipe Management and Electronic Batch Records in accordance with GAMP4 and FDA’s 21 CFR Part 11. The 12 Coating Pans were each provided with local Touch Screen MMIs. Operators download recipes and confirm actions via these local graphical interfaces. The recipes determine the set points & required parameters within the DCS. All batches are identified by unique Batch ID, the Batch Software only requires tags/items to indicate start and stop events within each phase. Routes are used to define a complete batch process and phases to define the individual operations of the process. Reports are executed at a route or phase level. An additional Server has been added to the network, operating with High Integrity software. This software provides a complete audit trail of all human interactions with batch production. Records such as system configuration changes, data entries, record modifications, and data deletion are maintained in the separate audit database.
The audit database is completely isolated from the batch record database. Any attempt to access the system or change or delete data is recorded in the audit trail. The software enforces the uniqueness of user identification codes and electronic signatures so that only personnel with the requisite permissions are able to make changes to the system. All time-stamp information is stored in both local time and UTC, ensuring data integrity and compliance with the FDA regulations. In addition to the Batch Reports the software provides a configurable report generator for Management Information which can then be securely transmitted to other personnel.
Inerting

IES Lean Systems Ltd. specialise in Inerting System Applications for centrifuges, vent headers, dryers and anywhere there is the possibility of hazard through ignition of solvent fumes. Particularly, as with centrifuges where the potential hazard is increased by the presence of static electricity generated by rotating machinery. Frequently the control provided as part of the OEM package can be improved both from a safety and centrifuge availability aspect by retrofitting.
Case Study Purge Sequence of Centrifuge Inerting in Bulk Pharmaceutical Application:
Purge to 2% Oxygen
Start Centrifuge
If Oxygen Exceeds 5% Alarm, Stop Feed
If Oxygen Falls to 2% Restore Feed
If Oxygen Exceeds 8% Flush with Nitrogen
Stop Centrifuge.